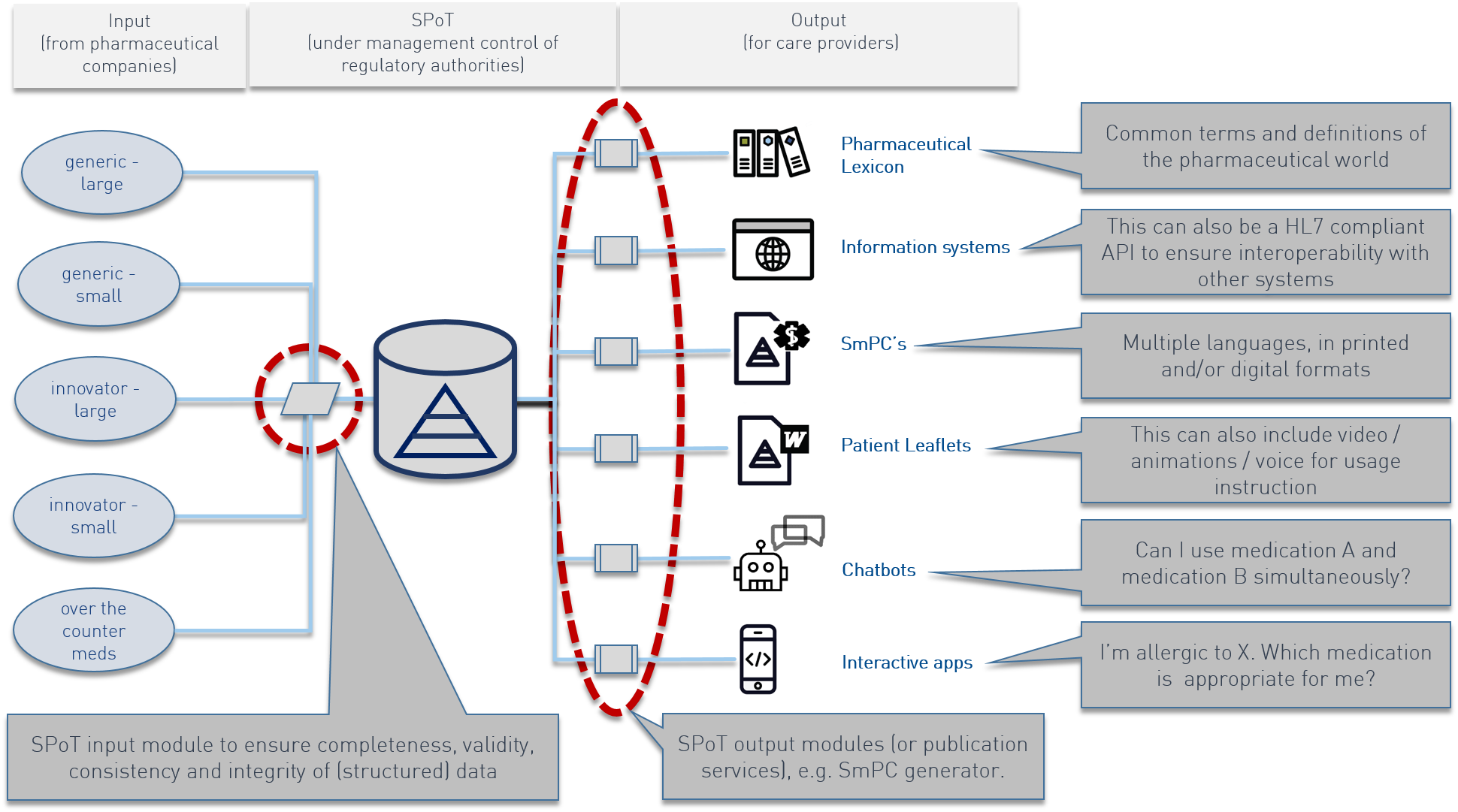
Having the right knowledge available at the right time is of the utmost importance for society. Especially in healthcare the right knowledge at the right time can be a matter of life and death. At any point in time, a healthcare stakeholder, whether it’s the pharmaceutical company, the healthcare provider, the patient or the government, must have access to the right knowledge at that time. In particular, improving proper medicine usage and medicinal therapy adherence requires a shared knowledge position on medicinal products. At brightpharma we refer to this shared knowledge position as the “Single Point of Truth” (SPoT).
SPoT
Single Point of Truth: always the same knowledge about medicine for everyone
The development and maintenance of medicinal product information is a complex process. The most important document that combines all knowledge about a medicinal productis the Summary of Product Characteristics (SmPC). The SmPC is a document that is supervised by the European Medicine Authority (EMA). In The Netherlands, the ‘College ter Beoordeling van Geneesmiddelen’ (CBG) is responsible for ensuring that the contents of these documents are available and accurately transcribed in the Dutch patient leaflets.
Pharmaceutical companies gather knowledge about their medicinal products in clinical studies and user experiences from daily practice. All this information is combined and summarized in the SmPC that is subsequently updated annually with the latest developments according to standard processes and legislation.
A key part of this process is the obligation of knowledge suppliers, generally the pharmaceutical companies, to supply the information in – EMA approved – regulatory documents (the so called QRD templates). Working with these templates is quite labor intensive and makes it necessary that in The Netherlands alone 350 people are employed at pharmaceutical companies to work on these dossiers, while at the regulatory agency CBG 275 people review the submitted materials almost on a daily basis.
One of the most important risks of the contemporary methods is that every individual pharmaceutical company can have its own interpretation of the rules. On top of that, the supervision by the regulatory agencies to ensure the correct application of the rules is done through human eyes, as the knowledge updates are currently not structured enough to be processed in an automatic way. A shared knowledge position is lacking. The possibility of a more systematic approach that is supported by IT therefore constitutes a great opportunity for improving the quality and efficiency for pharmaceutical companies and regulators.
It is with this opportunity in mind that brightpharma has developed the SPoT concept. A concept that has as its goal the delivery of high quality product information in an efficient and timely way to all healthcare stakeholders.
Key words
EMA – CBG – SmPC – SPC – patient leaflet – updates
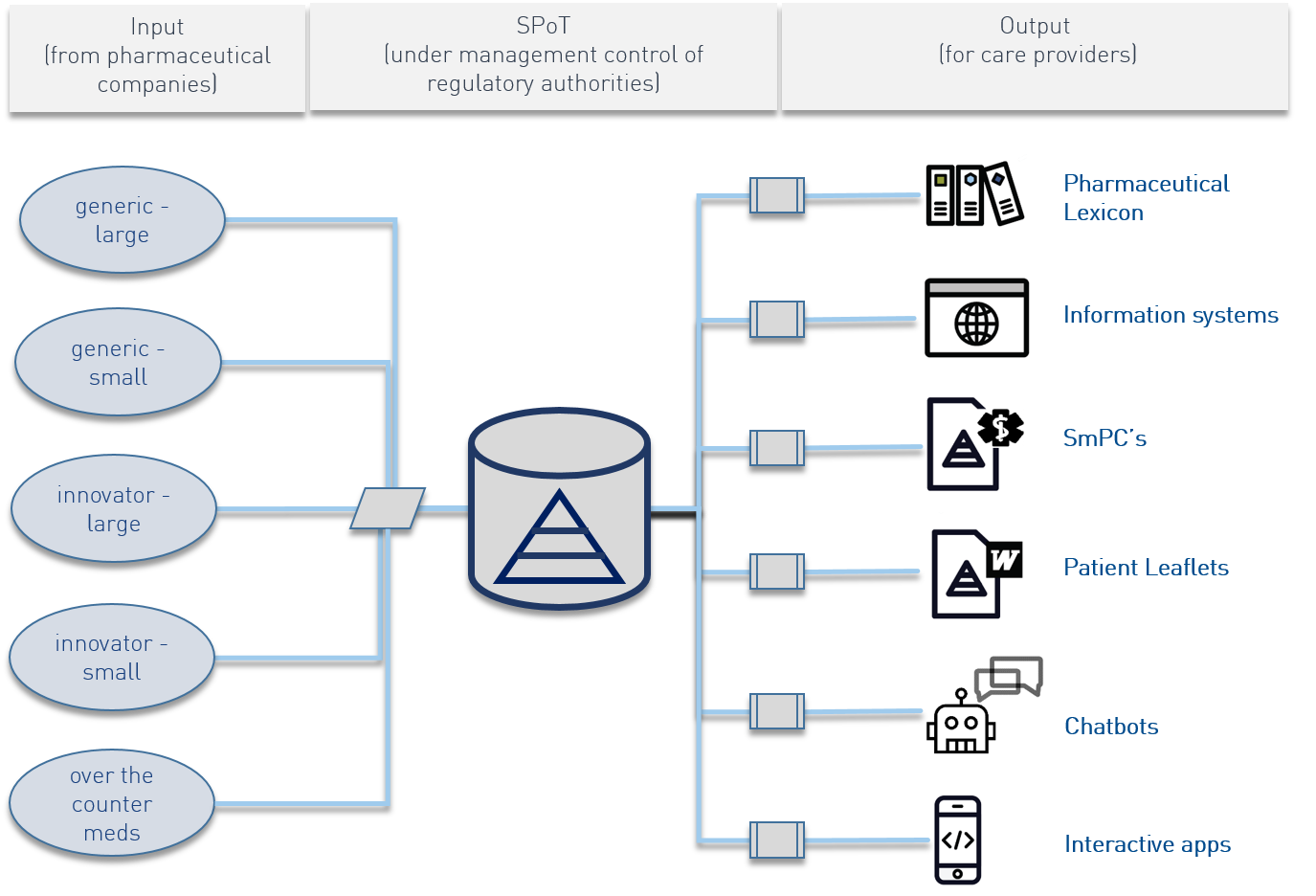
The SPoT concept is aimed at improving input of medicinal product information in terms of completeness, correctness, timeliness and consistency.
Consider the following example: the pharmaceutical form of a medicine (like a droplet, a tablet or an injection) can be mentioned a dozen times in a SmPC or patient leaflet. When this is the case, the pharmaceutical company also has to enter this text a dozen times in the regulatory QRD document. The regulator also has to review this text in a dozen places if something changes. This process is labor intensive and increases the possibility of errors/mistakes.
The SPoT creates an automated knowledge environment where all data only have to be entered once. The word droplet, tablet or injection becomes a single point of truth for the pharmaceutical form and is automatically inserted into the product information, labels and the patient leaflet where the pharmaceutical form is required, without the need for human interaction. At least 80% of a patient leaflet can be constructed automatically in such a way through SPoT. As a result, the use of SPoT decreases the risk of errors and the necessity of regulators to manually review 100% of all documents (some manual review will always remain necessary to a certain extent). The yearly updates by pharmaceutical companies similarly become much easier.
Key words
Standardization – harmonization – single entry – consistency
The SPoT environment also records the rules that certain information must adhere to.
For example: If a medicine has the pharmaceutical form “drink”, then the unit for this medicine will be expressed in milliliters or deciliters. A patient leaflet will never state that three 10 milliliter “tablets” should be taken daily. The rule dictates that the combination of tablets and milliliters is not allowed.
The SPoT ensures the product information is compliant to the many rules that regulators have set. By entering the data in the SPoT consistently and by guarding this input through automatic execution of rules, the quality and efficiency of the input are increased. As the SPoT is available 24/7, it is always up to date and requires minimal manual intervention. SPoT is the Google Maps of pharmacy!
In the SPoT-concept standardization of data, processes and rules regarding medicinal product information occurs, supported by a framework that identifies and records the meaning of the knowledge unambiguously. A lot of information elements regarding medicinal products are already encoded. Examples include pathology in ICD10-codes, the names of medicines in ATC-codes, side effects in MedDRA-codes and medical terminology in SNOMED-codes. Brightpharma adds the semantic standardization of, for example, OMG (Object Management Group) and classification systems, modelled in the knowledge modelling language cogNIAM.
As a result SPoT becomes the foundation of high quality product information and the starting point for patient-oriented information services and –products. These can be texts on patient leaflets or first issue information as well as digital information carriers like chatbots or virtual pharmacists.
Key words
Minimal manual intervention – structure – output independent – explicit rules
As soon as all data have been entered into the SPoT in a structured manner, the knowledge carriers can be utilized flexibly. For EMA or CBG this means that patient leaflets and SmPCs can be generated directly from the SPoT in different manifestations, for example as paper documents, as PDF or as html pages. For healthcare providers this means that the SPoT is the unambiguous information carrier for all communication materials (physical and digital) for the patient. For patients this means that patient leaflets can be delivered at different language levels, but always based on the same data from the SPoT.
The SPoT also creates a view across the borders of personalized medicine. Intelligence such as: “Which medicines in The Netherlands or Europe contain the additive sodium?”, or “Which medicines can have kidney damage as a side effect?” can be extracted swiftly and flawlessly from a well-structured SPoT.
Key words
Faster – more consistent – more accurate – more up to date – more goal oriented
It is clear that a SPoT is advantageous to all parties in the pharmaceutical ecosystem.
Pharmaceutical companies spend substantially less time and money on the processes for medicinal product information (new as well as revisions), as they enter data once in a controlled manner and can process updates more easily. The approval process with the regulator is easier and more efficient.
Regulators save time and money, as SPoT streamlines the approval process and requires less manual labor. After all structure and consistency do not have to be checked manually anymore.
Healthcare providers using SPoT can be certain that the data are complete, consistent, approved and up to date. This means that there is always a digital source of knowledge that is correct. That is why SPoT is also called the Single Point of Trust. Also, application of medicinal product information in a diversity of output formats, is much more easy.
Ultimately, the end user of a pharmaceutical product benefits greatly: as high-quality information is readily available to patients and healthcare providers, more time and financial resources are available for a personalized approach, personalized care and patient oriented innovation.